December 4, 2024 3pm ET: #SexCells: Investigating Parental Toxicant Exposures in the Etiology of Autism and Neurodevelopmental Disorders @ Harvard TH Chan
Via Zoom. Register here
October 25, 2024: Researcher meeting on research on parental exposures in the missing heritability of autism
This meeting is by invitation only. For more information please email jill.escher@gmail.com.
Escher Fund for Autism 2024 Grant Program
$100,000 grants available; deadline to submit expressions of interest: April 30, 2024
Pioneering a New Mammalian Model of Autism Spectrum Disorder via Germline Exposure
(A) Background: The Need for a Non-Genetic, Yet Heritable, Model of ASD
Autism spectrum disorder (ASD) is a strongly heritable mental disorder that has, paradoxically, proven to be only weakly genetic, with rare genetic errors accounting for about 10-14% of cases. While many in the field now presume the missing heritability of autism can be found in common variants, the evidence for this has been extremely weak, and inconsistent with the skyrocketing prevalence of this serious neurodevelopmental disorder.
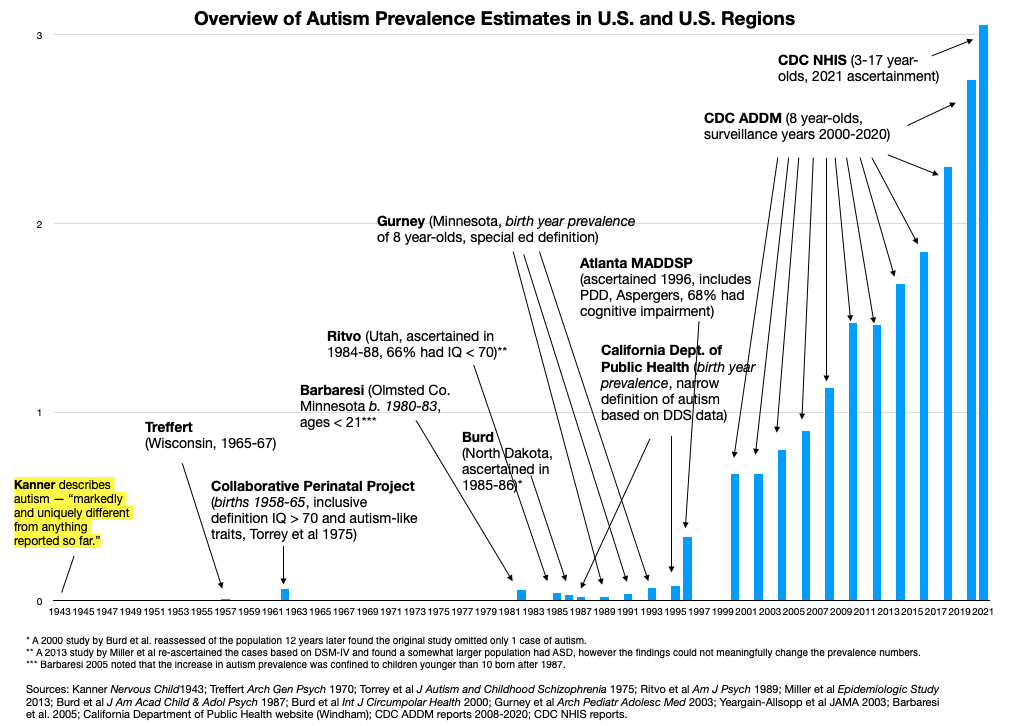
The Escher Fund for Autism takes a different approach. We are concerned that much of the missing heritability of autism, along with much of its sharply increasing prevalence (Figure 1), is more likely to be found in the under-researched realm of germ cell toxicology. In certain cases, molecular perturbations of the parental (F1) germ cell can result in the transcriptomic defects seen in offspring (F2) autism brains, with parallel impairments in F2 behaviors, without the existence of a de novo mutation.
Figure 1: Historical overview of studies of autism prevalence in the U.S. Autism prevalence began to noticeably increase with births in the late 1980s and early 1990s. Similar trends have been seen elsewhere, including western Europe, Australia, Japan and Israel.
This has important implications for animal models for autism. Most animal models use a mutation-based approach, which may have limited applicability outside of that particular syndrome. The field would benefit from development of new models that involve heritable dysregulation of early brain development seen in autism, but without a rare mutation driving that pathology. This grant program is designed to spur the creation of the first of such models.
The ultimate biological question — and why this model could be important for autism research — is creating dysregulation of gene expression in brain development in the F2 offspring of F1 exposed animals. Research suggests that autism is largely the result of abnormal gene expression during brain development (please see References in part E(6) below). But from where does this abnormal gene expression stem? We believe the answer may lie in the epigenome/chromatin of the parental germ cells.
In the modern world, human germ cells, egg and sperm and their precursors, do not arise in a pristine vacuum of biological perfection. Over the course of their development, germ cells can suffer any number of toxicological challenges, which in some cases may reshape their molecular programming and ultimately their blueprint for gene expression in the offspring. While the vast majority of exposures likely have little to no effect on germ cells, certain toxic exposures in certain windows of vulnerability may cause pathology in the next generation, as demonstrated widely in mammalian models.
___________________________________________________________
(B) Findings of Adverse Heritable Impact: The Inhalational General Anesthetic Sevoflurane
One acute yet very common toxic exposure that has repeatedly been shown in animal models to lead to neurodevelopmental abnormalities in offspring of exposed parents, via germline exposure, is the widely used inhalational anesthetic gas sevoflurane. Sevoflurane, a flourinated ether, was first used in the 1990s, was approved by the FDA in 1995, and has since risen to become the most commonly used halogenated anesthetic gas in the United States. According to one manufacturer, there have been more than a billion utilizations of sevoflurane since its introduction.
Studies on heritable impacts of sevoflurane encompass many findings that are consistent with phenomena observed in human autism:
Sevoflurane exposure can alter the transcriptional landscape in the exposed F1 germ cells.
The germline genes most affected by the exposure relate to neurodevelopment and neuronal functioning.
The brains of the offspring of exposed parents may exhibit abnormalities consistent with the germline errors.
The behaviors of the offspring, involving social, communication and repetitive behaviors can deviate from those of controls, and may be consistent with ASD.
The male offspring are affected to a much greater degree than the female offspring (which is consistent with one of the most puzzling yet durable findings in autism epidemiology). Also higher F2 littermate risk for pathology, due to perturbation of the pool of F1 germ cells.
Please review Section E, below, for an overview of the relevant research regarding this phenomenon.
__________________________________________________________
Figure 2: Example of sevoflurane inhalation anesthesia in a laboratory mouse. Source: Cesarovic et al. 2012.
(C) General Outline of Laboratory Work to Create a New Animal Model
This RFP seizes on these troubling observations to challenge researchers to lay the groundwork for a new human-relevant non-genetic but heritable rodent model of autism, involving the following elements:
(1) Organism. An appropriate strain of rat or mouse.
(2) F1 Exposure(s). A plan for exposure of male and/or female mice (F1) to sevoflurane (see Figure 2 for an example), which may include:
— A range of clinically relevant doses. Please note that in humans sevoflurane concentrations are significantly higher when used for infants and young children.
— A range of clinically relevant time frames (eg, reflective of 1, 2, 3 or more surgical procedures). The exposure(s) can be delivered any time in the pre-conception life or lives of the F1 parent(s), from the fetal (if the F0 gestating dam is exposed) to the F1 adult periods. Please note that early life germline exposures may be more vulnerable than later exposures, though the sevoflurane studies show germline impact throughout pre-conception lifespan.
—Optional exposure to other drugs and anesthetics to more accurately reflect clinically relevant human exposures in surgery, imaging or dental care.
—It is not necessary to observe F1 post-exposure long-term behaviors, but this may be included. For example it may be relevant in the case of F1 exposures in very early development (for example, to development of the broader autism phenotype).
—Attention to a “tipping point” for dose/timing/repetition. We seek a study design capable of determining points at which risks for F2 autism traits in neuropathophysiology and/or behavior cross a clinically relevant threshold. In other words, how, across a range of exposure, does the study determine if the F1 exposure crosses a threshold relevant to risk for F2 human autism spectrum disorder?
(3) F1 Mating. A plan for, upon sexual maturity, mating of the exposed F1s with exposed and/or naive F1 mates.
(4) F1 Germline. A plan for analysis of F1 sperm and/or egg. Possible endpoints include cellular pathology, epigenetic/chromatin alterations, impacts on imprinted genes, and/or genetic damage. In males, the plan may also include sperm concentration, morphology, motility; impacts on Sertoli cells. In males, explain whether the tissues to be tested are spermatogonial stem cells, mature spermatozoa, and/or other types. In females, attention to impact on oocyte maturation and reserve is desirable.
(5) F2 Behaviors. A plan for observation of behavioral outcomes of F2 offspring, which may include social, communication, and repetitive behaviors, and sensory processing, all with sex-specific analysis. Ideally a plan would also observe pathology that is often co-morbid with autism, including but not limited to anxiety, abnormal socio-sexual behaviors, and hyperactivity.
(6) F2 Brain. A plan for observation of brain and brain development abnormalities in F2 offspring, with sex-specific analysis. Does the F2 brain exhibit impaired neuron development, proliferation, migration and/or synaptogenesis, in one or more regions? Defects in inhibitory circuits and E/I balance, or other brain functions relevant to autism?
_________________________________________________________
(D) Requirements for the Expressions of Interest
(1) Title. A title for the project.
(2) Investigators. Basic information about the investigators. Please attach NIH biosketch.
(3) Basic hypothesis. Your overall approach, in 200 words or less.
(4) Outline. Brief outline of the study following part (C) above, providing rationales for all choices. Please briefly explain the methods to be used.
(5) Implications for autism research. Assuming some F2 offspring exhibit brain and behavior consistent with ASD, please briefly explain what might be the implications of your animal model for (a) further model development; (b) understanding of autism pathogenesis; (c) efforts toward prevention of autism (which could including anything from pre-medications to protect germline, to clinical practice modifications, to paternal sperm testing); and (d) efforts toward treatment of ASD.
(6) Funding. Brief description of funding requested (up to $100,000 per grant) and how the funds would be used, and timeline anticipated, with reasonable line items. Due to the limited size of these grants, which are intended to generate pilot data on which larger grants may then be based, please reference possible leveraging of existing work or studies. If the work is to be done in phases, please describe those phases and the expected budgets for each. Note: For our grant programs, we have a policy to not pay indirect costs.
These expressions of interest are due April 30, 2024. Submission of PDF files are preferred. Please email expressions of interest (questions prior to that are also welcome) to jill.escher@gmail.com.
Authors of submissions meeting review criteria will be invited to submit more detailed information as may be needed. We anticipate making grants in the latter part of the year. We reserve the right to make changes to this RFP at any time for any reason, and changes will be posted at EscherFund.org. If no expressions of interest meet review criteria, we reserve the right to modify the 2024 grant program.
_______________________________________________________
(E) Background Research and References (Each section offered in chronological order)
(1) Mammalian Models: Studies Demonstrating Adverse Heritable Neurodevelopmental Impacts of Halogenated Anesthetic Gases, via Germline Exposure
Taken together, the studies offer evidence that agents of general anesthesia, with sevoflurane being the most studied, can induce molecular changes in germline, changing transcription of key brain development genes and inducing adverse neurodevelopmental outcomes in progeny, particularly males.
Chalon, J., Tang, C.K., Ramanathan, S., Eisner, M., Katz, R. and Turndorf, H., 1981. Exposure to halothane and enflurane affects learning function of murine progeny. Anesth Analg, 60(11), pp.794-797.
Small study finding maternal line F2 generation of halothane exposed gestating F0 female mice to be “significantly slower than control mice throughout the training” on all days of testing and all configurations of a maze test. The authors concluded that the impaired learning in the F2 “suggests that the anesthetic agent may have caused a genetic aberration” in the exposed mothers’ fetal eggs.
Tang CK, Chalon J, Markham JP, Ramanathan S, Turndorf H. Exposure of sires to enflurane affects learning function of murine progeny. Anesth Analg 1984; 63: 729-730.
The same lab reported that enflurane caused impaired learning function in the generation born of the exposed germ cells, this time later-stage sperm instead of early-stage eggs. The researchers remarked that it “seems likely that spermatogenetic changes, caused by enflurane, are associated with genetic alterations” that affected the pups’ brain development.
Ju, L.S., Yang, J.J., Morey, T.E., Gravenstein, N., Seubert, C.N., Resnick, J.L., Zhang, J.Q. and Martynyuk, A.E., 2018. Role of epigenetic mechanisms in transmitting the effects of neonatal sevoflurane exposure to the next generation of male, but not female, rats. Brit. J Anaesthesia, 121(2), pp.406-416.
Sub-clinical concentrations of sevoflurane were administered to male and female neonate rat pups and the directly exposed animals and their progeny were examined Using the elevated plus maze and the Morris water maze tests, it was found that the F0 and F1 male animals exhibit abnormal behaviors in both tests, indicating increase in anxiety and impairment in spatial memory. These behavioral abnormalities were associated with changes in gene expression of the potassium chloride cotransporter 2 (Kcc2). Kcc2 expression is reduced by 20-40% in the hypothalamus and less than 20% in the hippocampus of F0 and F1 male animals compared to unexposed controls. DNA methylation in the promoter of the Kcc2 gene was examined in sperm of exposed males and hypothalamus and hippocampus of offspring males, and found to increase significantly in the six CpG sites examined after sevoflurane exposure. These data suggest that the down-regulation of Kcc2 gene expression and increased promoter CpG methylation in the F1 hypothalamus is associated with the increased in Kcc2 promoter CpG methylation of the F0 sperm. Kcc2 is a central nervous system (CNS) neuron-specific chloride potassium symporter localized at excitatory synapses that is essential for synaptic inhibitions, synaptic spin morphogenesis and neuroplasticity. Mutations or changes in in Kcc2 expression are involved in many neurological diseases including brain trauma, epilepsies, autism and schizophrenia. These findings suggest that sevoflurane could induce a nongenetic effect in early-stage germ cells, causing some sex-specific brain and behavioral abnormalities in the next generation, even when used at low concentrations.
Ju, L.S., Yang, J.J., Xu, N., Li, J., Morey, T.E., Gravenstein, N., Seubert, C.N., Setlow, B. and Martynyuk, A.E., 2019. Intergenerational effects of sevoflurane in young adult rats. Anesthesiology, 131(5), pp.1092-1109.
In addition, administration of sevoflurane to young adult rats (with more mature germ cells) resulted in similar, though not identical, abnormalities in parental germ cells and in male offspring of exposed sires and dams. Notably, the lab’s experiments suggested that compared to the somatic cells, the germ cells are more sensitive to the deleterious effects of sevoflurane, raising the possibility that male offspring may be affected even when the anesthesia level/duration is insufficient to induce significant abnormalities in exposed parents.
Xu, N., Lei, L., Lin, Y., Ju, L.S., Morey, T.E., Gravenstein, N., Yang, J. and Martynyuk, A.E., 2020. A methyltransferase inhibitor (decitabine) alleviates intergenerational effects of paternal neonatal exposure to anesthesia with sevoflurane. Anesth Analg, 131(4), p.1291.
Studies by the same group found that expression of DNA methyltransferase 3a and 3b (Dnmt3a and Dnmt3b, enzymes that catalyze the transfer of a methyl group to DNA) in the hypothalamus of F1 animals was increased by more than 40% compared to unexposed control males. When the animals were treated with Decitabine, a methyltransferase inhibitor, prior to sevoflurane exposure, the expression of Dnmt3a, Dnmt3b, and Kcc2 in the hypothalamus of F1 animals were similar as unexposed control animals and the animals exhibit normal behaviors. These data suggest that DNA methyltransferase activity might be involved in the response to sevoflurane exposure at the Kcc2 locus. In the future, it would be interesting to perform genome wide analyses of changes in gene expression and DNA methylation in this system.
Chastain-Potts, S.E., Tesic, V., Tat, Q.L., Cabrera, O.H., Quillinan, N. and Jevtovic-Todorovic, V., 2020. Sevoflurane exposure results in sex-specific transgenerational upregulation of target IEGs in the subiculum. Molecular Neurobiology, 57, pp.11-22.
Another lab recently performed experiments with some similar aims but looking only at the offspring brain as an endpoint rather than the parental germ cells or offspring behaviors. After exposing neonatal female rats to sevoflurane, they bred the females and found their offspring’s brains exhibited epigenetic abnormalities, including reduced DNA methylation in hippocampal neurons and upregulation of Arc and JunB mRNA expression in males born to exposed females, an effect linked to functional decline in learning and memory. This effect was sexually dimorphic, again only noted in the male progeny.
Wang, H.L.V., Forestier, S. and Corces, V.G., 2021. Exposure to sevoflurane results in changes of transcription factor occupancy in sperm and inheritance of autism. Biol. Reprod., 105(3), pp.705-719.
Demonstrated a molecular basis for neurodevelopmental pathology in offspring of sperm of sons of pregnant mice exposed to sevoflurane. Gestating mice were exposed at day E12.5 of embryonic development for 2 hours, as this is the time when the germline of the exposed fetus is fully demethylated and may be more susceptible to environmental exposures. Adverse behavioral defects were observed in more than 38% of the directly exposed F1 males, including sociability deficits and increased anxiety as measured by the three-chamber sociability test, bedding shredding and marble burying tests. By outcrossing the F1 males to unexposed females for two generations, sevoflurane was found to have both “intergenerational” (F2 derived from exposed germline) and “transgenerational” (F3 derived from germline never exposed to sevoflurane) actions. In fact, 44–47% of the F2 and F3 showed the same behavioral problems as the F1 males (females were not tested). Behavioral phenotypes correlate reduced neonatal brain size and weight. However, the brain size and weight differences were not apparent in the mature adult mice. The inter- and transgenerational inheritance through the male germ cells was confirmed by Assay for Transposase-Accessible Chromatin sequencing (ATAC-seq) experiments in sperm of the F1 and F2 generations, which showed a dramatic recruitment of TFs to enhancer sequences of genes found to be associated with ASD, including Arid1b, Ntrk2, and Stmn2.
Ju, L.S., Zhu, J., Brant, J.O., Morey, T.E., Gravenstein, N., Seubert, C.N., Vasilopoulos, T., Setlow, B. and Martynyuk, A.E., 2023. Intergenerational Perioperative Neurocognitive Disorder in Young Adult Male Rats with Traumatic Brain Injury. Anesthesiology, 138(4), pp.388-402.
After paternal head trauma and Sevoflurane exposure, male but not offspring exhibited brain and behavior abnormalities, reduced Nr3c1 expression in F1 male, but not female, hippocampus was accompanied by corresponding Nr3c1 promoter hypermethylated CpG sites in F0 spermatozoa and F1 male, but not female, hippocampus.
Ju, L.S., Zhu, J., Morey, T.E., Gravenstein, N., Seubert, C.N., Setlow, B. and Martynyuk, A.E., 2024. Neurobehavioral abnormalities in offspring of young adult male rats with a history of traumatic brain injury. Journal of Neurotrauma. Offspring of rate sires with a history of a moderate TBI that involved craniectomy under sevoflurane anesthesia for 40 min, develop sex-specific neurobehavioral abnormalities, even in the absence of direct social interaction between the sire and the offspring. F1 male offspring of TBI sires exhibited abnormalities in all behavioral tests, while their F1 female counterparts had abnormal pre-pulse inhibition responses only. F1 male offspring of TBI sires also had reduced mRNA levels of hippocampal Nr3c1 and Nr3c2, as well as hypothalamic and hippocampal Bdnf, whereas increases in inflammatory markers were more profound in F1 females.
(2) Studies Demonstrating Adverse Germline Impact of Halogenated General Anesthesia (selection)
Coate, W. B., Kapp, R. W. Jr, & Lewis, T. R. 1979. Chronic exposure to low concentrations of halothane-nitrous oxide: reproductive and cytogenetic effects in the rat. Anesthesiology, 50, 310–318.
Land, P.C., Owen, E.L. and Linde, H.W., 1981. Morphologic changes in mouse spermatozoa after exposure to inhalational anesthetics during early spermatogenesis. Anesthesiology, 54(1), pp.53-56.
Arena, A.C. and Pereira, O.C.M., 2002. Neonatal inhalatory anesthetic exposure: reproductive changes in male rats. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 133(4), pp.633-640.
Arena, A. C., & Pereira, O. C. 2002. Neonatal inhalatory anesthetic exposure: reproductive changes in male rats. Comparative Biochemistry and Physiology. Toxicology & Pharmacology, 133, 633–640.
Ceyhan, A., Cincik, M., Bedir, S., Ustun, H., Dagli, G., & Kalender, H. 2005. Effects of exposure to new inhalational anesthetics on spermatogenesis and sperm morphology in rabbits. Archives of Andrology, 51, 305–315.
Wang, L.J., Wang, X.H., Sun, H.J. and Xu, B., 2008. Effects of inhaled anesthetics on human sperm motility in vitro. Zhonghua nan ke xue= National Journal of Andrology, 14(4), pp.338-342.
Xu, X.L., Pan, C., Hu, J.X., Liu, X.T., Li, Y.F., Wang, H., Chen, Y.B., Dong, H.Y., Dai, T.J. and Xu, L.C., 2012. Effects of isoflurane inhalation on the male reproductive system in rats. Environmental toxicology and pharmacology, 34(3), pp.688-693.
Kaymak, C., Kadioglu, E., Coskun, E., Basar, H. and Basar, M., 2012. Determination of DNA damage after exposure to inhalation anesthetics in human peripheral lymphocytes and sperm cells in vitro by comet assay. Human & experimental toxicology, 31(12), pp.1207-1213.
Kaya, Z., Sogut, E., Cayli, S., Suren, M., Arici, S., Karaman, S. and Erdemir, F., 2013. Evaluation of effects of repeated sevoflurane exposure on rat testicular tissue and reproductive hormones. Inhalation Toxicology, 25(4), pp.192-198.
Tang, X.N., Yao, W., Yao, H.X., Zhang, Y. and Yue, J., 2020. Influence of isoflurane exposure for 15 consecutive days on ovarian function in adult female mice. Current Medical Science, 40, pp.1177-1181.
Qingzhen, L., Yuping, H., Lidong, Z. and Junfeng, Z., 2023. Effects of exposure to the inhalational anaesthetic sevoflurane on the male reproductive system in rats. Veterinary Medicine and Science.
Zanin, M., Varela Junior, A.S., Bonel Acosta, I., Anastacio Da Silva, E., Gehrcke, M.I. and Corcini, C.D., 2023. Acute exposure to isoflurane impairs sperm parameters in mice. Drug and Chemical Toxicology, pp.1-8.
(3) Study Demonstrating Widespread Epigenetic Impacts Post-Surgery in Human Sperm
Donkin, I., Versteyhe, S., Ingerslev, L.R., Qian, K., Mechta, M., Nordkap, L., Mortensen, B., Appel, E.V.R., Jørgensen, N., Kristiansen, V.B. and Hansen, T., 2016. Obesity and bariatric surgery drive epigenetic variation of spermatozoa in humans. Cell Metabolism, 23(2), pp.369-378.
The only study to date to examine epigenetic impacts on human sperm following surgery. Found very significant changes in sperm DNA methylation both one week and one year after bariatric surgery.
(4) Epidemiology Consistent with The Hypothesis
While there are no epidemiological studies to date regarding heritable impacts of sevoflurane there are several studies that are consistent with the idea that F1 medical interventions and exposures raise risk for F2 autism or ADHD.
Schieve, L.A., Drews-Botsch, C., Harris, S., Newschaffer, C., Daniels, J., DiGuiseppi, C., Croen, L.A. and Windham, G.C., 2017. Maternal and paternal infertility disorders and treatments and autism spectrum disorder: findings from the study to explore early development. JADD, 47, pp.3994-4005.
Unexpected links to maternal fertility-related conditions (which may have involved surgical intervention) ["While the magnitude of the aORs among second or later births was near or above 3.0 for four conditions (blocked tubes, PCOS, endometriosis, and uterine or related problems), indicating strong associations with ASD, aORs were markedly lower among first births. For two conditions (blocked tubes and PCOS), the findings were null among first births and for the other two conditions (endometriosis and uterine and related problems) aORs (1.8 and 1.6, respectively) were 45–71% lower among first births and the confidence intervals for these estimates overlapped 1.0."].
Kioumourtzoglou, M.A., Coull, B.A., O’Reilly, É.J., Ascherio, A. and Weisskopf, M.G., 2018. Association of exposure to diethylstilbestrol during pregnancy with multigenerational neurodevelopmental deficits. JAMA Pediatrics, 172(7), pp.670-677.
Grandchild impacts of gestational diethylstilbestrol (DES) exposure found significantly elevated odds for ADHD in the grandchildren; 7.7% vs 5.2% if diethylstilbestrol was taken during the first trimester of pregnancy. It seems possible that because DES daughters have a higher risk for surgery (eg, on the cervix), that perhaps that mediating variable could be related to increased offspring risk.
Ejlskov, L., Wulff, J.N., Kalkbrenner, A., Ladd-Acosta, C., Fallin, M.D., Agerbo, E., Mortensen, P.B., Lee, B.K. and Schendel, D., 2021. Prediction of autism risk from family medical history data using machine learning: A national cohort study from Denmark. Biol Psych Global Open Sci, 1(2), pp.156-164.
A machine-learning study looking at family history of many different medical conditions found "The highest risk score group had 17.0% ASD prevalence and a 15.3-fold (95% confidence interval, 14.0–17.1) increased ASD risk compared with the lowest score group, which had 0.6% ASD prevalence. In contrast, individuals with a full sibling with ASD had 9.5% ASD prevalence and a 6.1-fold (95% confidence interval, 5.9–6.4) higher risk than individuals without an affected sibling." May point to medical interventions and not just medical conditions as a mediating risk factor.
Xiao, J., Gao, Y., Yu, Y., Toft, G., Zhang, Y., Luo, J., Xia, Y., Chawarska, K., Olsen, J., Li, J. and Liew, Z., 2021. Associations of parental birth characteristics with autism spectrum disorder (ASD) risk in their offspring: a population-based multigenerational cohort study in Denmark. Int. J. Epidemiol., 50(2), pp.485-495.
Offspring of mothers or fathers with adverse birth characteristics had about 31–43% higher risk for ASD. Parents born very preterm (<32 weeks) marked a nearly 2-fold increase in ASD risk in their children. Notable that preterm infants are at sharply increased risk for surgery and other early life medical interventions.
Schendel D, Ejlskov L, Overgaard M, Jinwala Z, Kim V, Parner E, Kalkbrenner AE, Acosta CL, Fallin MD, Xie S, Mortensen PB, Lee BK. 3-generation family medical histories of mental, neurologic, cardiometabolic, birth defect, asthma, allergy, and autoimmune conditions associated with autism. medRxiv [Preprint]. 2024 Feb 13:2023.11.03.23298042. doi: 10.1101/2023.11.03.23298042. PMID: 37961212; PMCID: PMC10635276.
Increased adjusted hazard ratios for ASD (26,840 cases; 1.6% of births) were observed for almost all individual mental disorder-family member type combinations yet for fewer non-mental disorder-family member type combinations. aHRs declined with diminishing degree of relatedness between the index person and family member for some disorders, especially mental disorders. Variation in aHR magnitude by family member sex (e.g., higher maternal than paternal aHRs) or side of the family (e.g., higher maternal versus paternal half sibling aHRs) was more evident among non-mental than mental disorders. Co-occurring ID in the family member or the index person impacted aHR variation.
Kravets ME, Klebanoff MA, Keim SA. Associations between maternal exposure to surgery or pregnancy exposure to fluorinated anesthetics and children’s cognitive development and educational outcomes. Journal of Developmental Origins of Health and Disease. 2023;14(2):199-208. doi:10.1017/S2040174422000472.
While animal models suggest an intergenerational impact of modern halogenated anesthetics, the germline impact of old-school surgical anesthesia (eg, ether, nitrous oxide, ethylene, trichloroethylene, cyclopropane, chloroform, vinyl ether, somnoform, alcoform, anesthol, ethyl chloride) has not been explored, and perhaps should not be suspected considering low rates of neurodevelopmental disorders in children born in the 1960s (autism rates did not notably uptick until births in the mid 1980s). Based on a new study using data from the Collaborative Perinatal Project of the 1960s, and funded by the Escher Fund, the next-generation impact of these exposures seem to be mixed. Maternal outpatient surgical history was used as a proxy for exposure to general anesthesia. Among the findings: Maternal surgery in early childhood was associated both with being in a special school or not in school (adj OR=1.42; 95% CI 1.02, 1.98) and with slightly better cognitive ability across childhood (e.g., WISC IQ (adj β=0.59; CI 0.13, 1.04) (especially among boys)). Maternal surgery in puberty was associated with slightly lower IQ (adj β = –0.42; CI –0.79, –0.05) and poorer spelling at age 7.The study also looked at the proximate impact of specific gaseous anesthetic agents, including fluorinated agents, during the child's in utero development (mostly in the 1960s, so halothane, penthrane, fluromar). Prenatal exposure to fluorinated anesthetics was associated with lower performance IQ at age 7.
(5) Studies Finding Abnormal Epigenetic Markers in Sperm of Autism Fathers
Feinberg, J.I., Bakulski, K.M., Jaffe, A.E., Tryggvadottir, R., Brown, S.C., Goldman, L.R., Croen, L.A., Hertz-Picciotto, I., Newschaffer, C.J., Daniele Fallin, M. and Feinberg, A.P., 2015. Paternal sperm DNA methylation associated with early signs of autism risk in an autism-enriched cohort. Int J Epidemiol., 44(4), pp.1199-1210.
A small study finding that DNA from the sperm of men whose children had early signs of autism shows distinct patterns of regulatory tags that could contribute to the condition. While the study did not explore paternal exposure history, it found 193 differentially methylated regions (DMRs) where the presence or absence of DNA methylation was statistically related to autism scores based on the Autism Observation Scale for Infants (AOSI) in their offspring. Interestingly, 24% of AOSI-associated DMRs are also found in the cerebellum of autistic individuals, lending support to the idea of causal relationship between epigenetic alterations in sperm and in adult tissues.
Garrido, N., Cruz, F., Egea, R.R., Simon, C., Sadler-Riggleman, I., Beck, D., Nilsson, E., Ben Maamar, M. and Skinner, M.K., 2021. Sperm DNA methylation epimutation biomarker for paternal offspring autism susceptibility. Clinical Epigenetics, 13, pp.1-13.
A small study on sperm of fathers who have children with ASD observed a set of 805 DMRs in sperm that could potentially act as a biomarker for paternal offspring autism susceptibility. “Ancestral or early-life paternal exposures that alter germline epigenetics are anticipated to be a molecular component of ASD etiology.”
Feinberg, J.I., Schrott, R., Ladd-Acosta, C., Newschaffer, C.J., Hertz-Picciotto, I., Croen, L.A., Daniele Fallin, M., Feinberg, A.P. and Volk, H.E., 2023. Mol. Psych., pp.1-11. Epigenetic changes in sperm are associated with paternal and child quantitative autistic traits in an autism-enriched cohort.
Findings suggest paternal germline methylation is associated with autistic traits in 3-year-old offspring. These prospective results for autism-associated traits, in a cohort with a family history of ASD, highlight the potential importance of sperm epigenetic mechanisms in autism.
(6) Transcriptomic Studies in Autism (selection)
Perturbed transcriptional pathways are seen broadly in autism cases, both in studies based on peripheral tissues and post-mortem autism brains. The transcriptional dysregulation of autism seems to occur notwithstanding the absence of any detected genetic mutations.
Voineagu, I., Wang, X., Johnston, P., Lowe, J.K., Tian, Y., Horvath, S., Mill, J., Cantor, R.M., Blencowe, B.J., & Geschwind, D.H. 2011. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 474, 380-4 doi:10.1038/nature10110
Gupta, S., Ellis, S.E., Ashar, F.N., Moes, A., Bader, J.S., Zhan, J., West, A.B., & Arking, D.E. 2014. Transcriptome analysis reveals dysregulation of innate immune response genes and neuronal activity-dependent genes in autism. Nat Commun 5, 5748 doi:10.1038/ncomms6748
Ansel, A., Rosenzweig, J.P., Zisman, P.D., Melamed, M. and Gesundheit, B., 2017. Variation in gene expression in autism spectrum disorders: an extensive review of transcriptomic studies. Frontiers in neuroscience, 10, p.601.
Wang, P., Zhao, D., Lachman, H.M. and Zheng, D., 2018. Enriched expression of genes associated with autism spectrum disorders in human inhibitory neurons. Translational psychiatry, 8(1), p.13.
Gazestani, V.H., Pramparo, T., Nalabolu, S., Kellman, B.P 795 ., Murray, S., Lopez, L., Pierce, K., Courchesne, E., & Lewis, N.E. 2019. A perturbed gene network containing PI3K-AKT, RAS-ERK and WNT-β-catenin pathways in leukocytes is linked to ASD genetics and symptom severity. Nat Neurosci 22, 1624-1634 doi:10.1038/s41593-019-0489-x
Quesnel-Valli.res, M., Weatheritt, R.J., Cordes, S.P., & Blencowe, B.J. 2019. Autism spectrum disorder: insights into convergent mechanisms from transcriptomics. Nat Rev Genet 20,51-63 doi:10.1038/s41576-018-0066-2
Hughes, H.K., Rowland, M.E., Onore, C.E., Rogers, S., Ciernia, A.V. and Ashwood, P., 2022. Dysregulated gene expression associated with inflammatory and translation pathways in activated monocytes from children with autism spectrum disorder. Translational psychiatry, 12(1), p.39.
Additional note re the GABAergic system:
Gamma-aminobutyric acid A (GABAA) receptors are ligand-gated ion channels responsible for mediation of fast inhibitory action of GABA in the brain. Many autism studies have implicated disruptions GABAergic system during neurodevelopment. Sevoflurane and related chemicals enhance the function of GABAA receptors, the most abundant fast inhibitory neurotransmitter receptor in the CNS. They also have modest to strong effects on other ion channels, including glycine receptors, neuronal nicotinic receptors, 5-HT3 receptors, glutamate receptors and the two pore potassium channels. Isoflurane, desflurane and sevoflurane all enhance the amplitude of responses to low concentrations of GABA and prolong the duration of GABA mediated synaptic inhibition. GABA receptors exist in germ cells, which may suggest a mechanism. However, other mechanisms may also be at play that result in an altered transcriptional landscape in germ cells.
(7) General Commentary Regarding This Phenomenon
Escher, J. and Ford, L.D., 2020. General anesthesia, germ cells and the missing heritability of autism: an urgent need for research. Environmental Epigenetics, 6(1), p.dvaa007.
Martynyuk, A.E., Ju, L.S., Morey, T.E. and Zhang, J.Q., 2020. Neuroendocrine, epigenetic, and intergenerational effects of general anesthetics. World J. Psychiatry, 10(5), p.81.
Martynyuk, A.E., Ju, L.S. and Morey, T.E., 2021. The potential role of stress and sex steroids in heritable effects of sevoflurane. Biol. Reprod., 105(3), pp.735-746.
Escher, J., Yan, W., Rissman, E.F., Wang, H.L.V., Hernandez, A. and Corces, V.G., 2022. Beyond genes: germline disruption in the etiology of autism spectrum disorders. JADD, 52(10), pp.4608-4624.
Ju, L.S., Morey, T.E., Seubert, C.N. and Martynyuk, A.E., 2023. Intergenerational Perioperative Neurocognitive Disorder. Biology, 12(4), p.567.
Escher, J. May 2023 presentation, Germ Cell Toxicant Exposures in the Etiology of Offspring Neurodevelopmental Pathology, at UCLA Lundquist Institute
_________________________________________________________
Questions about the Escher Fund for Autism 2024 grant program may be directed to: jill.escher@gmail.com
The Escher Fund for Autism is a donor-advised fund at Schwab Charitable.
We fund:
• Autism research, particularly in the field of non-genetic inheritance
• Autism-serving programs such as schools, recreation providers, camps, family support networks, adult day programs and housing
Past grantees include, among many others:
University of Bristol • Child Health and Development Survey • University of Copenhagen • UCSF • UCSD • UC Davis • Rockefeller University • Brown University • UCLA • Autism Speaks • Linkoping University • University of Chicago • Stanford University • Florida State University • University of Florida • North Carolina State University • Colorado State University • University of Colorado • Columbia University • Keystone Symposia • Gordon Research Conferences • International Society for Autism Research • Environmental Mutagenesis & Genomics Society • Society of Toxicology • Ben Gurion University • University of Syracuse • Washington State University • Nationwide Children's Hospital • University of Bradford • Harvard University • Emory University • University of Nevada, Reno • American Society of Andrology
Morgan Autism Center • Friends of Children with Special Needs • Magical Bridge Foundation • Mental Health and Autism Insurance Project • Via Services • Monterey Bay Horsemanship Therapy • Magical Bridge Foundation • Camping Unlimited • The Ed Asner Family Center • Life Services Alternatives • Autism Society San Francisco Bay Area • National Council on Severe Autism • Together for Choice • Shared Adventures • Friendship Circle
______________________________________________________________________________________________
ARCHIVES FROM PAST GRANT PROGRAMS
Autism Research Grants: Earlier research-oriented RFPs can be found at GermlineExposures.org
Autism Services Grants: We do not have currently have an open grant program for autism/DD services. We provide grants mainly to San Francisco Bay Area nonprofits serving the needs of the local autism community.
2021 Request for Proposals: Adult Programs
Severe Autism and I/DD Adult Day or Recreation Program Innovation Grants
Grants up to $100,000 available for programs in Santa Clara and/or Santa Cruz Counties
Deadline to apply: Friday, December 17, 2021 at 11:59 p.m.
Background:
Escher Family Fund (EFF) is a donor-advised fund at Silicon Valley Community Foundation focused on supporting autism-related programs and research.
The purpose of this grant program is to support projects that help address what has been called “the Grand Canyon of service gaps” — the lack of viable day or recreational program options for adults with severe forms of autism. We define severe forms of autism as individuals with significant intellectual disabilities, poor adaptive functioning, and challenging behaviors such as property destruction, disruptive behaviors or vocalizations, elopement, aggression and/or self-injury. These individuals often require 1:1 or even 1:2 or higher client:staff ratios. For the purposes of this grant, clients must have aged out of school-funded services.
Through this grant program, EFF seeks to support nonprofit organizations in Santa Clara County and/or Santa Cruz County in providing innovative solutions for day programming and/or meaningful engagement for these severely disabled adults.
Programming can take place at centers, the community, or even in the home, on weekdays, or weekends. It can take place individually or in groups. We encourage creativity and out-of-the-box person-centered thinking. For example:
A school or other facility with a fenced playground or recreational area (including pools) can use grant funds to offer space to adults (with family or staff) when appropriate, for example after the close of the school or work day, or on weekends.
A program can provide in-home services offering music and dance lessons.
A program can embellish staff wages with incentives to attract and retain staff capable of working with this challenging population.
A program can purchase a van that will enable it to take clients on hikes, to parks, etc.
Capital improvements such as safe spaces, safety fencing, swings and sensory-friendly amenities that will enable a program to serve severely autistic and I/DD clients.
A program that inspires work-like efforts such as trash pick-up, or skill building.
These are just examples, we encourage innovative thinking.
Eligibility
Organizations must serve Santa Clara and/or Santa Cruz County. Organizations headquartered outside the two-county region may apply but should demonstrate significant service to these areas or partner with a local organization that does.
New or existing organizations may apply.
Programs must be insured but need not be licensed by Community Care.
Organizations can submit a cooperative application, in partnership.
Organizations must have a 501(c)(3) designation or have a fiscal sponsor with a 501(c)(3) designation.
Organizations must not discriminate based on race, color, national origin, citizenship status, creed, religion, religious affiliation, age, gender, marital status, sexual orientation, gender identity, disability, veteran status or any other protected status.
Organizations with religious affiliations will be considered for funding only if the project for which they seek support addresses the needs of the wider community without regard to religious beliefs.
Ultimately, we hope that these grants can demonstrate successful models that can be expanded and/or replicated so as to serve a larger number of adults, across a broader region. Grant recipients will need to provide detailed outcome reporting to ensure accountability and inform future grantmaking.
The minimum amount of each grant will be $5,000 and the maximum amount will be $100,000. The grant review process will be expedited and only one application may be submitted per organization. Deadline for submission is December 17, 2021 at 11.59pm. Applicants may be asked follow-up questions to clarify any questions raised by the application. Applications for lower grant amounts may receive preference over those for higher amounts. Awards will be announced no later than March 1, 2022. Funds will be disbursed once the organization is project-ready.
This RFP may be modified at any time.
Funding will not be provided for:
Budget shortfalls or fundraising events
Previously planned or long-term campaigns
Funding gaps due to internal organizational emergencies (such as office vandalism, resignation of an executive director or sudden loss of funding)
Expenses that should under law be covered by government agencies such as SARC.
How to apply:
Please submit your application by December17, 2021, 11:59pm PST, here in an attached Word document. The total word count should not exceed 3,000, and it should include the following information:
BACKGROUND
Please include:
Contact person and title
Contact email
Contact phone
Contact address
Organization website
Description of organization’s mission, services, and geographic service area
Verify the organization is a 501(c)(3) nonprofit
History of this organization and/or organization leaders serving this particular target population
PROPOSAL
Please include:
Basic overview of proposal (up to 1,000 words)
Timeline for project
How the organization measures impact. How do you know you have been successful in your services? Are there any specific quantitative or qualitative data that you will try to capture? Explain how you will capture total client service hours served by the grant.
Characteristics of the population to be served
Handling of transportation-related issues
FINANCIALS; MAXIMIZATION OF GOVERNMENT AND OTHER FUNDING
Please include:
This program is not intended to substitute for funding that under law should be provided by San Andreas Regional Center (SARC) or other publlc funding programs such as IHSS, WPCS, county mental health, or others. A component of your grant program should ensure absolute maximization of SARC and other funding for your severely autistic clients: such as negotiated rates, health & safety waivers; SARC vendor codes for items like personal aides, respite, behavioral support and START services. Your proposal must address this directly: how will you maximize your clients’ use of public funding.
Any leverage of existing facilities or government facilities (eg, can the County of Santa Clara Parks and Rec provide a program site?), and/or expertise
Any liability or worker’s comp issues you foresee
Amount requested (from $5,000 to $100,000)
Budget for project, with line items for use of all grant funds
REPORTING
Please state that you are willing to provide reporting both 6 months and 12 months after receipt of funds. For larger grants this timeline may be extended at the discretion of the EFF.
Thank you for your interest in this grant program. Questions about this RFP may be submitted here.